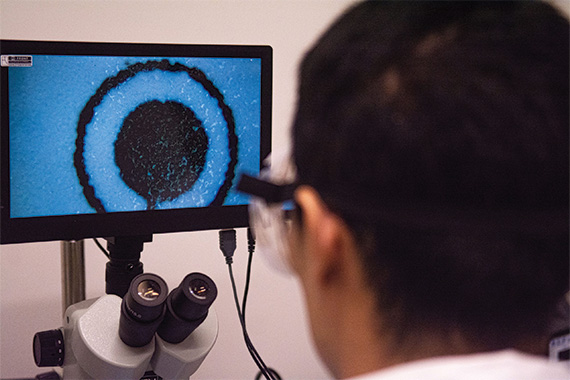
Within the rapidly evolving fields of healthcare and technology, many questions revolve around the consequences of newly enacted laws about health artificial intelligence. Regulatory frameworks are changing to ensure the ethical, safe, and effective integration of artificial intelligence in healthcare as the technology becomes more and more essential to medical operations including diagnosis, treatment, and patient care. About privacy, data protection, accountability, and the wider influence on healthcare practices, these new rules seek to address relevant concerns. Many parties are negotiating the complex junction of innovation and regulation, including healthcare providers, AI developers, and regulatory agencies. This raises important concerns regarding the direction of AI in the healthcare industry.
Industry Perspectives on AI Regulations
The healthcare landscape is on the brink of a transformative shift with the introduction of new AI regulations by the Health and Human Services (HHS), set to be enforced in 2025.
The Office of the National Coordinator for Health IT (ONC) has unveiled comprehensive rules, compelling AI developers to provide enhanced transparency about their algorithms. Industry reactions are a mixed bag, with major groups like the Coalition for Health AI applauding the move. The coalition, including tech giants Google and Microsoft, sees the regulations as striking a delicate balance, introducing standards without becoming overly prescriptive.
However, amidst the positive reception, questions linger regarding the collaboration between ONC and the FDA, which also regulates AI-enabled medical devices. Some express concerns about potential overlaps and a desire for differentiated treatment of products already under FDA regulation. The scope of the rules prompts queries about liability, particularly concerning individual clinicians tasked with determining AI trustworthiness. Industry experts stress the need for a structured process similar to addressing failures in conventional medical devices, urging clarity and a seamless interaction between regulatory bodies.
READ ALSO: Questions about new health AI rules
Liability and Clarity Concerns
The ONC’s reliance on individual clinicians to assess the trustworthiness of AI algorithms raises the issue of liability. The industry calls for a systematic approach akin to established processes for handling medical device failures. Additionally, questions arise about the precise algorithms covered by the rules, emphasizing the need for more clarity.
Despite these challenges, the ONC asserts a broad scope, encompassing models influencing care delivery beyond direct involvement in clinical decision-making, such as those supporting supply chains. As the healthcare sector grapples with these evolving regulations, the agency remains open to public feedback, underscoring a commitment to refining and improving the rules collaboratively.
READ ALSO: ‘We will make his life miserable’: GOP promises bruising confirmation for Pentagon policy pick