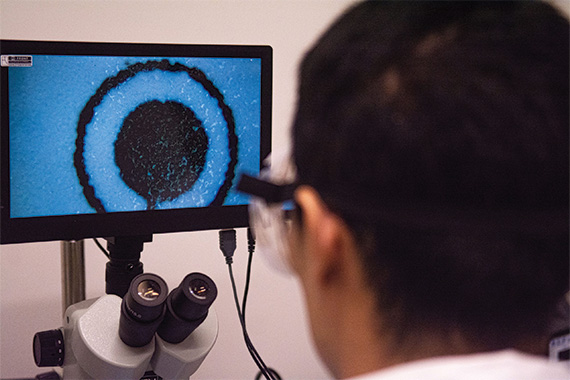
In the aftermath of the 2021 safety recall on Philips breathing devices, alarming revelations have surfaced regarding potential risks associated with the replacement machines distributed to customers. An investigation led by ProPublica and Pittsburgh Post-Gazette sheds light on these newfound concerns.
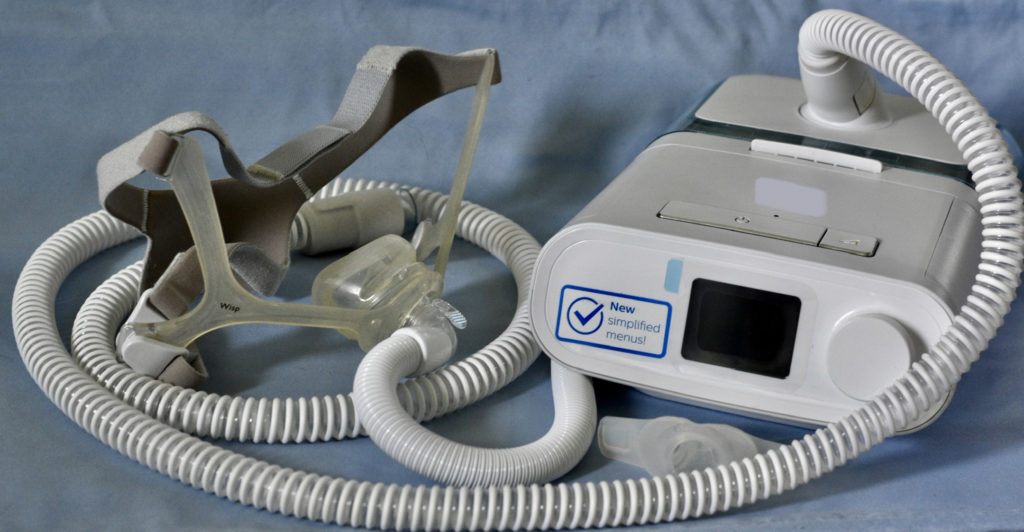
Post-Recall Risks Pose Threat to Users
Philips, a leading manufacturer of medical devices, faced a significant safety recall in 2021 related to one of its widely used breathing devices.
Despite efforts to address the initial safety issues, recent findings indicate that the replacement machines provided to consumers might introduce new and potentially dangerous problems. Ali Rogin delves into the details of the ongoing investigation, uncovering the scope and implications of these unforeseen risks.
The collaborative effort aims to provide a comprehensive understanding of the evolving safety landscape and the potential impact on users relying on these essential medical devices.
ProPublica and Pittsburgh Post-Gazette Investigate Ongoing Safety Issues
Debbie Cenziper, a prominent figure in the joint investigation conducted by ProPublica and Pittsburgh Post-Gazette, shares insights into the latest developments surrounding the Philips breathing device controversy.
Stay tuned as we unravel the intricacies of this investigation, exploring the implications for users and the steps being taken to address the post-recall risks associated with Philips breathing devices.