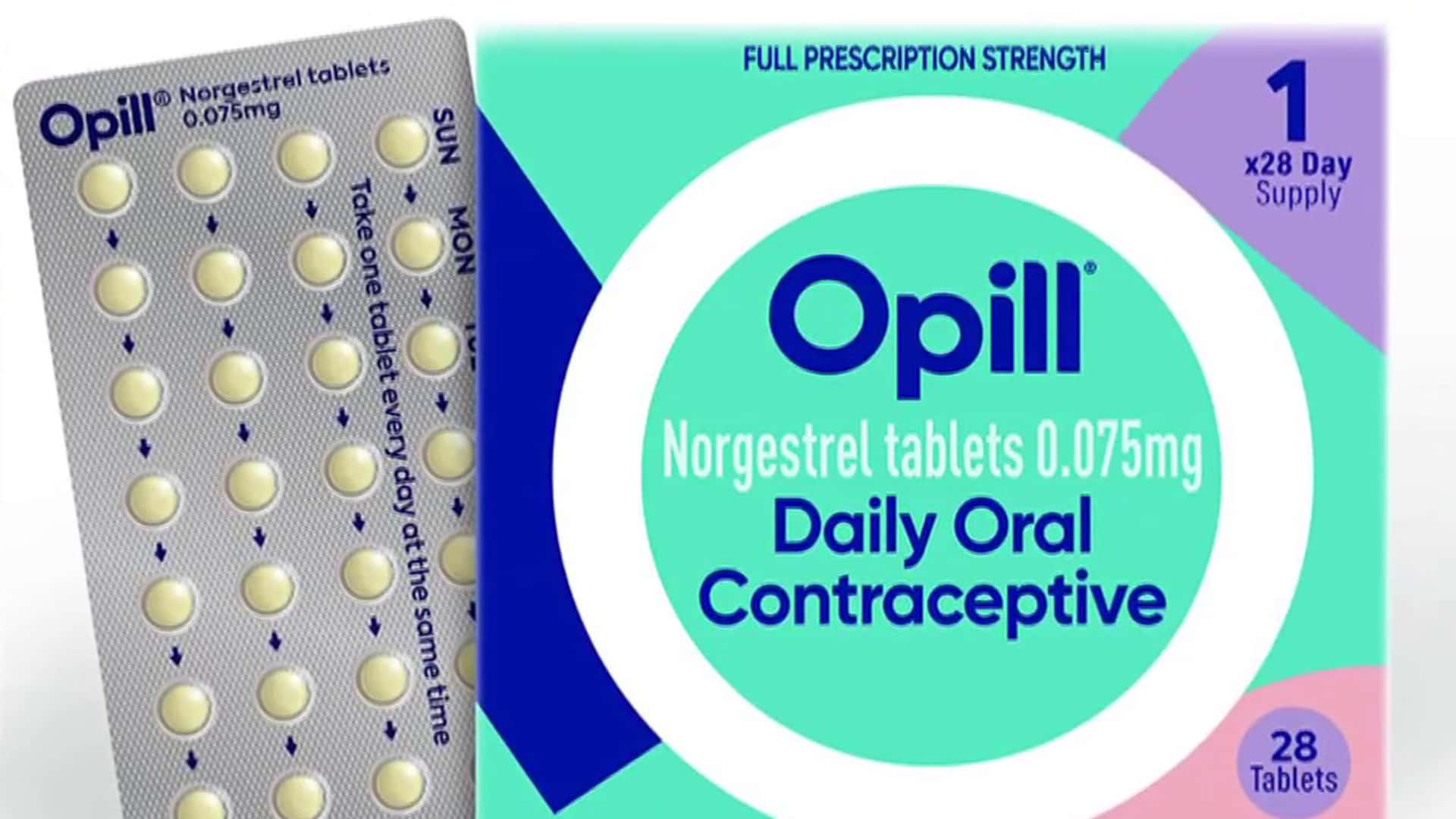
A decades-old contraception pill must be marketed without a doctor’s prescription, according to federal health experts on Wednesday, clearing the path for the potential authorization of initial over-the-counter contraception in the United States.
Perrigo Pharmaceuticals’ proposal to place its once-daily medicine on store racks among eye drops and allergy medications was approved by the FDA advisors’ panel with a unified vote. After a two-day discussion on whether women might utilize the pill successfully and securely without a doctor’s surveillance, a suggestion was made. This summer, a final FDA ruling is anticipated.
Opill, a medication made by Perrigo, is expected to be the first contraception pill to leave the drugstore counter if the agency complies with the non-binding advice. Sales might start later this year, the business claimed, if approved.
The other experts expressed their general confidence in the capability of women of all ages to use the medication properly without first consulting a healthcare professional.